Sidnee Pinho joins ClearView as the Chief Operating Officer
Several members of leadership team will be joining the discussion with global healthcare leaders at ISPOR Europe
Principal Ryan Tubman and Head of Analytics Mike Roy present a poster at the annual meeting of the American College of Epidemiology
Principal Charles Matthews will be attending to engage in collaboration and networking
Key guiding principles for effective forecasting, common pitfalls, and how to use a forecast to develop better strategy
Presented at the 2019 BIO International Convention in Philadelphia, this talk by Sam Ulin, Managing Director, examines the drivers of value for innovators, with a focus on the technologies and applications that attracted significant funding in 2018
At BIO International Convention in Philadelphia on June 3, 2019, Managing Director Sam Ulin will give lead the Super Session talk, "What’s Next: The Landscape of Innovation in 2019 and Beyond"
As genetic testing becomes more accessible, how are companies incorporating these technologies into their strategy?
RWE has become increasingly important in supporting supplemental or conditional drug approvals. Where is the space headed?
Managing Directors Phil Kenner and Seth Berman will be attending this springtime partnering conference
What are the implications of the DHHS' new plan to overhaul prescription drug rebates for federal beneficiaries?
The ClearView leadership team will be attending this industry-leading healthcare investment symposium
Launching a new therapy is a complex endeavor. We will review the various activities key to a successful drug launch.
How can flexibility in clinical development can be incorporated at various points along an asset’s life cycle
Navigating new challenges of market access and reimbursement for new pharmaceuticals in today's market
Recent changes in the orphan drug market access landscape and implications for pharmaceutical developers
Ketan Kapadia joins ClearView as a Managing Director
Distinct ways that medtech companies can incorporate digital health technologies into their portfolios
Building a well thought-out plan to establish evidence of safety and of clinical utility to establish trust in a new platform
Technical and transactional assessments required to identify the right type of partnership for a biotech’s asset or platform and overall business needs
This second installment of our “Growing Up Biotech” series identifies the key factors around a pivotal question posed when launching your product: to partner or not to partner?
In the first white paper in our series “Growing Up Biotech”, we discuss the importance of communicating a compelling product story early in a biotech’s development.
PAGs play an increasingly important role in the drug development and regulatory process. Companies that truly want to be patient-centered should engage these groups early, often, and enthusiastically.
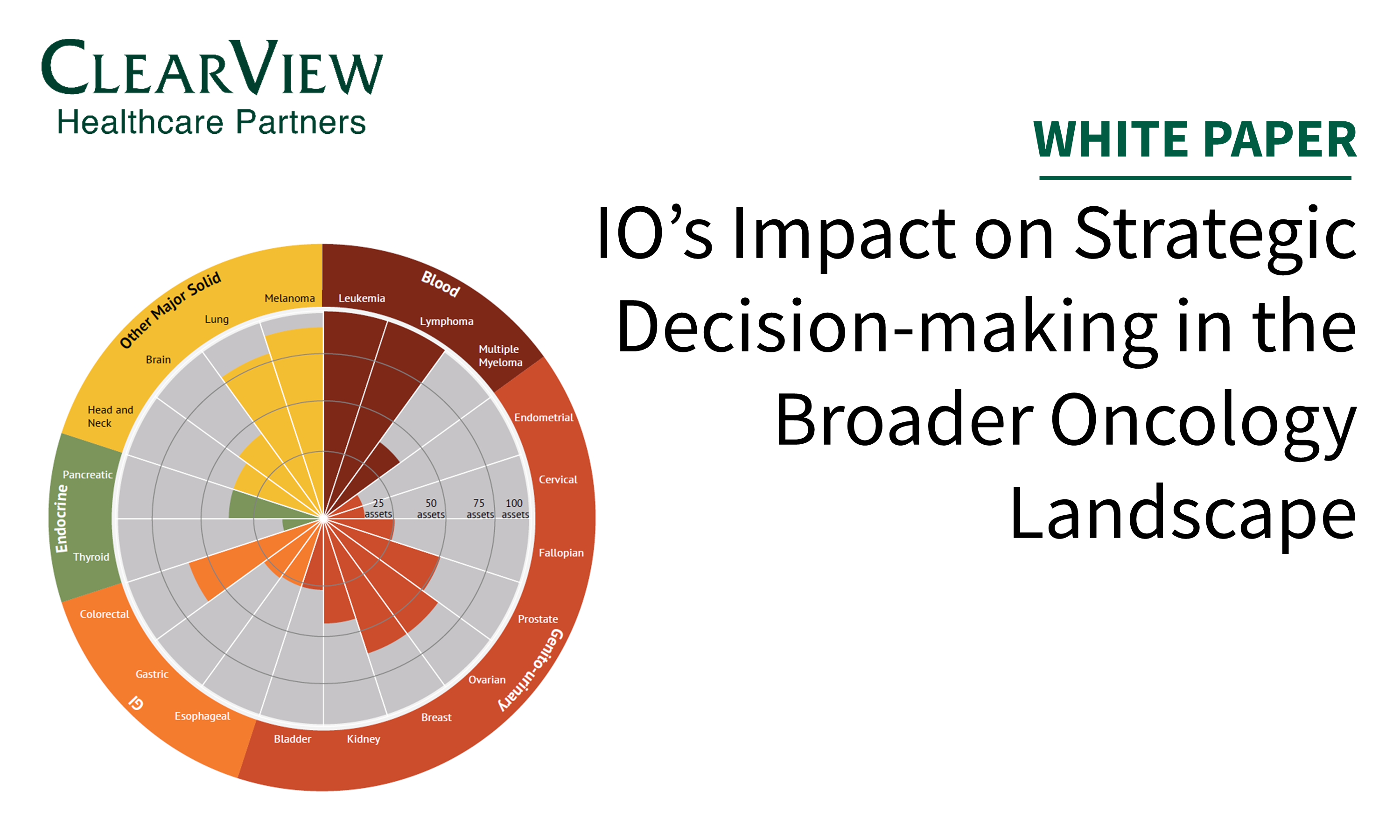
In this article published in In Vivo, we review key strategic considerations across clinical development, market access, and post-launch commercialization important for oncology drug development.
What are the clear areas of opportunity and the unique challenges associated with orphan disease drug development to help companies be best prepared to succeed in this exciting, but nuanced, space?
Informed drug development in an era of “precision medicine” requires a clear understanding of the risks and benefits that accompany the pursuit of companion diagnostics, a difficult objective given the complexity and rapid evolution of the field.